2024.01.01
Happy new year
Here is the first paper from our group for 2024. This first paper is one of the last ones from joint work with our esteemed colleague Vitalij K. Pecharsky whom we sadly lost about a year ago and makes us grateful for all the interaction with him and collaboration with colleagues.

2024.02.01
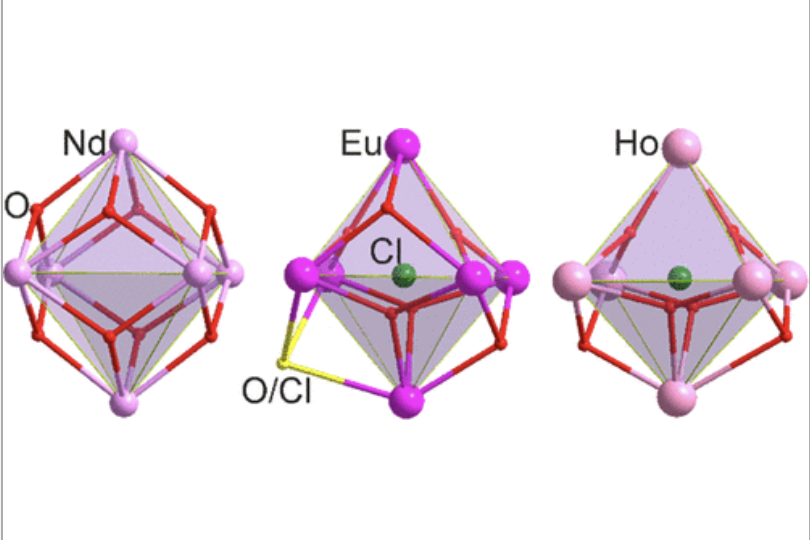
Plutonium chemistry
Plutonium chemistry is complex and there is a lot to learn, if we want to develop safe nuclear reactors and control the waste.
Looking at rare earth elements as stand-ins can help us to make informed guesses and decision. Read more in the most recent publication from our and the Rogers lab:
2024.02.10
A new indide
The structures of ternary rare earth intermetallics appear often at first sight complex, but can many times be understood as intergrowths of well-known structure types.

Read more from the publication here: The crystal and electronic structure of RE23Co6.7In20.3 (RE = Gd–Tm, Lu): A new structure type based on intergrowth of AlB2– and CsCl-type related slabs
2024.02.20
A remarkable bioester
Ever wondered why 2-ethylhexyl laureate is used in personal care products like moisturizer lotions hair shampoo, makeup, sunscreen? Small angle neutron scattering gives an answer:

Read the full study here: Small-Angle Neutron Scattering Insights into 2-Ethylhexyl Laurate: A Remarkable Bioester
2024.03.01
Honeycomb motifs
An article in honor of Vitalij K. Pecharsky – when looking for honeycomb motifs in intermetallics our team discovered two new isostructural series of intermetallics: La4Co4M (M = Sn, Sb, Te, Pb, Bi) and La3Ni3M (M = Al, Ga):
2024.04.01
Ionic liquid assisted microwave synthesis
Combining ionic liquids with microwave heating allows to make otherwise difficult to obtain solid solutions of perovskites. Making a solid solution of BaSnO3 and BaZrO3 allows for band gap tuning, an important aspect for photocatalysis.

Read more about it here: Synthesis and Exploration of Barium Stannate–Zirconate BaSn1–xZrxO3 (0 ≤ X ≤ 1) Solid Solutions as Photocatalysts
2024.04.05
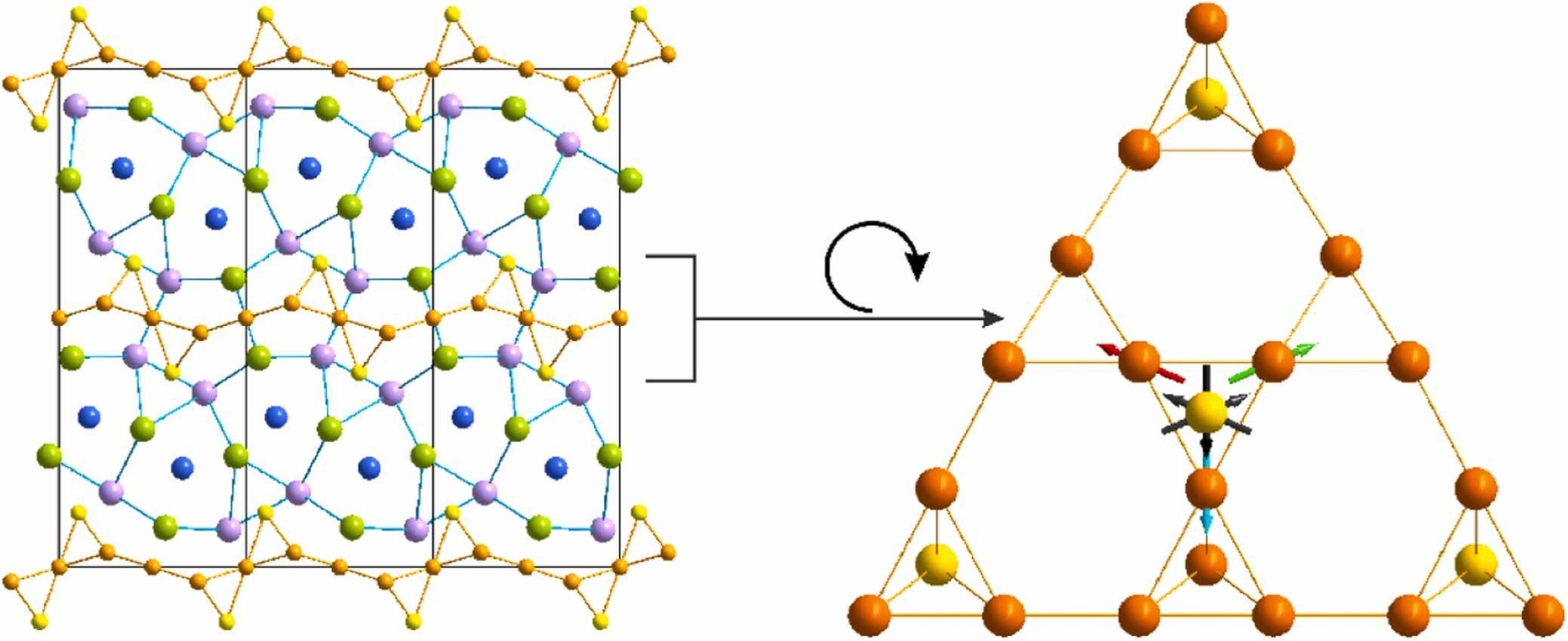
Hedgehog spin-vortex state
Realizing a hedgehog spin-vortex state is art in chemistry and physics! But it is not only aesthetic – combining magnetic frustration with electrical conductivity can allow simultaneous charge and spin manipulation, which is crucial for the design of next generation electronic devices.

Read more here: Making a Hedgehog Spin-Vortex State Possible: Geometric Frustration on a Square Lattice
2024.04.10
Surface matters!
Nanoparticles have stunning properties. Would you have ever imagined that the luminescence of nanoparticles could actually come from surface species? It is time to revise commonly accepted views.

Read the full J. Am. Chem. Soc paper here: Manipulation of Luminescence via Surface Site Occupation in Ln3+-Doped Nanocrystals
2024.04.20
Improving our understanding of the structure of ionic liquids
Read about our collaborative investigation of three nonhalogenated ionic liquids in terms of their bulk and electro-interfacial nanoscale structures using small-angle neutron scattering (SANS) and neutron reflectivity (NR) to understand the structure of ionic liquids:

2024.06.10
The power of molten salts in achieving a sustainable and safe steady energy supply
Professor Anja-Verena Mudring has received a DKK 60 million grant from the Novo Nordisk Foundation’s 2024 Challenge Programme for her project, “SMARTER – Salt Melts for Advanced Reactor Technology and Energy Research.” This initiative will tackle the critical challenges of providing society with an economical, safe, stable, and sustainable energy solution.
As the world seeks to meet the ambitious goals of the Green Deal, nuclear energy is being reconsidered. Despite concerns over reactor accidents, nuclear waste, and weapons proliferation, a promising technology known as the molten salt reactor (MSR) was conceptualized as early as the 1960s. MSRs, which confine nuclear fuel within molten salts, offer a potentially safer alternative to traditional reactors. However, significant challenges remain in making this technology viable.
AVM highlights the unique aspects of MSRs, particularly their non-proliferation benefits and enhanced safety features: “The nuclear reactor we aim to investigate is different from current reactors. We want to develop a system where the nuclear material cannot be used to produce weapons. The byproducts of our proposed reactors would make any weapon unstable.”
Through the Novo Nordisk Foundation’s support, Professor Mudring will bring together a team of experts from Denmark and the United States to address these fundamental issues. The research does not advocate for or against nuclear energy. Instead, it focuses on bridging these knowledge gaps to enable more 3 of 5 informed decisions. The insights gained from this research will also have broader applications, including heat management and thermal energy storage using molten salts. Therefore, the project will contribute to a sustainable future, even if nuclear energy is not adopted.