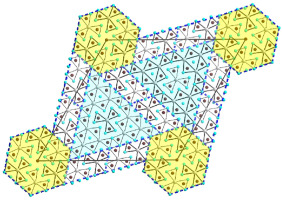
The Mudring group and collaborators have described a new class of open framework materials (OFMs). OFMs, such as zeolites, contain voids in their structures that give them attractive functionalities including catalysis, gas storage, separation and ion exchange. The framework structures of practically all known inorganic OFMs are restricted to tetrahedral building blocks, such as silicate (SiO44-). In their paper, however, the authors describe their discovery of a whole new class of OFMs with exclusively octahedral building blocks forming the open framework. The octahedral building blocks of these new compounds are reminiscent of the zero-dimensional units that form polyoxometalates (POMs), which are known for their interesting magnetic properties. Repeating those units in three dimensions in an open framework make the authors’ new OFMs interesting for new applications in magnetics, like spintronics for the green transition.
The authors’ approach to synthesising these new materials is key. The use of ionic liquids – that act as the reaction medium, mineralizer, and templating agent – allows for the formation of the open framework instead of dense phases that form by using traditional solvents.
Read the article here.




 Can natural products be a basis for green lighting technology? 8-Hydroxyquinoline is a natural product found in the fungus Cortinarius subtortus and the plant Allium stipitatum (aka the Persian shallot). 8-Hydroxyquinoline and its derivatives can act as antiseptics and disinfectants, have anti-inflammatory properties, and are heavily researched as antineurodegeneratives, antidiabetics, and anticancer agents in medicine. Its structure renders 8-hydroxyquinoline a good emitter material from the natural pool for light emission. Unfortunately, rapid proton transfer quenches the emission. This can be overcome by complexation with metal ion, such as in tris(8-hydroxyquinolinato) aluminium, which is a compound widely used in OLEDs. However, metal ions such as aluminium are suspected to cause diseases like Alzheimer’s. Thus, metal-free alternatives are more desirable. In a collaboration of chemists, physicists, and chemical engineers we now have developed 8-hydroxyquinoline into a metal-free, natural product platform molecule for true green lighting.
Can natural products be a basis for green lighting technology? 8-Hydroxyquinoline is a natural product found in the fungus Cortinarius subtortus and the plant Allium stipitatum (aka the Persian shallot). 8-Hydroxyquinoline and its derivatives can act as antiseptics and disinfectants, have anti-inflammatory properties, and are heavily researched as antineurodegeneratives, antidiabetics, and anticancer agents in medicine. Its structure renders 8-hydroxyquinoline a good emitter material from the natural pool for light emission. Unfortunately, rapid proton transfer quenches the emission. This can be overcome by complexation with metal ion, such as in tris(8-hydroxyquinolinato) aluminium, which is a compound widely used in OLEDs. However, metal ions such as aluminium are suspected to cause diseases like Alzheimer’s. Thus, metal-free alternatives are more desirable. In a collaboration of chemists, physicists, and chemical engineers we now have developed 8-hydroxyquinoline into a metal-free, natural product platform molecule for true green lighting.